Levels of Evidence
Levels of evidence are assigned to studies based on the methodological quality of their design, validity, and applicability to patient care. The combination of these attributes gives the level of evidence for a study. In nursing, the system for assigning levels of evidence is often from Melnyk & Fineout-Overholt's 2011 book, Evidence-based Practice in Nursing and Healthcare: A Guide to Best Practice. The Levels of Evidence below are adapted from Melnyk & Fineout-Overholt's (2011) model.

About Levels of Evidence and the Hierarchy of Evidence: While Levels of Evidence correlate roughly with the hierarchy of evidence (discussed elsewhere on this page), levels of evidence don't always match the categories from the Hierarchy of Evidence, reflecting the fact that study design alone doesn't guarantee good evidence.
About Levels of Evidence and Strength of Recommendation: The fact that a study is located lower on the Hierarchy of Evidence does not necessarily mean that the strength of recommendation made from that and other studies is low--if evidence is consistent across studies on a topic and/or very compelling, strong recommendations can be made from evidence found in studies with lower levels of evidence, and study types located at the bottom of the Hierarchy of Evidence. In other words, strong recommendations can be made from lower levels of evidence.
For example: a case series observed in 1961 in which two physicians who noted a high incidence (approximately 20%) of children born with birth defects to mothers taking thalidomide resulted in very strong recommendations against the prescription and eventually, manufacture and marketing of thalidomide. In other words, as a result of the case series, a strong recommendation was made from a study that was in one of the lowest positions on the hierarchy of evidence.
Hierarchy of Evidence: Qualitative and Quantitative
Quantitative Evidence Pyramid
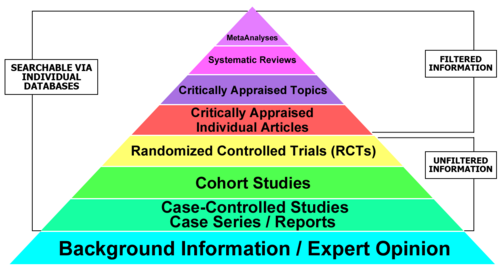
Qualitative Evidence Pyramid
